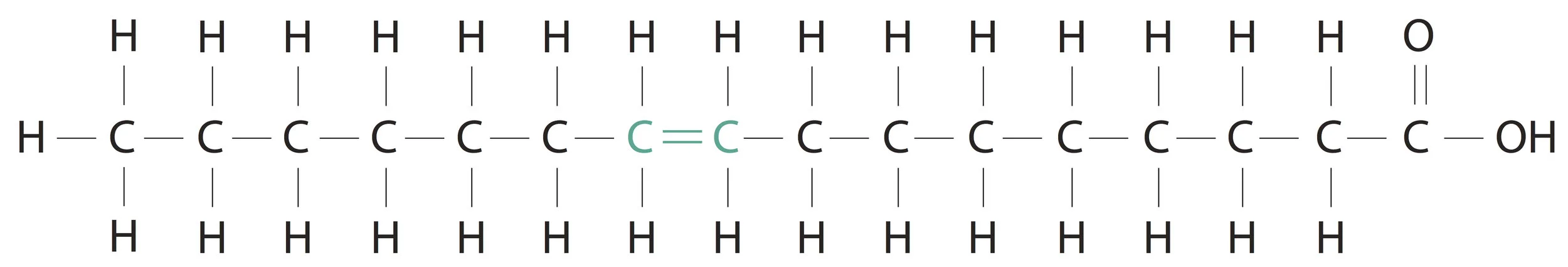Fatty Acids - The building blocks of lipids
The quest for understanding lipids has puzzled us all since first year of med school. We tried our best to understand, most of us didn’t. Then we decided not to complicate things further and did our best mugging them up. Now we are left with nothing but a bare idea about a few lipids like HDL, LDL, VLDL, triglycerides, cholesterol and their normal serum ranges, just for the sake of knowing what statins to start on our grandparents whenever their labs go wrong.
Are these lipids that hard? YES.
Lipids are complicated. But the amount of knowledge a medical graduate has to retain for a good practice, is just little. This little can even be broken down to ‘petite’ when we understand what lipids really are. The fact is, these lipids aren’t anything new. We’ve learnt them at school.
But we missed the link.
We missed the link between the chemistry of fatty acids we had learnt in school and the biochemistry of lipids that we now learn in college.
In fact, these fatty acids are the building blocks of lipids!
Where do we start? We call them lipids because they do not dissolve in water. This hydrophobic property of lipids is the reason for most of their functions and the structures they take. We shall start from the simplest forms of lipids and slowly move on to their complex structures and then to their metabolism. Let's start with a basic question:
So what is a fat?
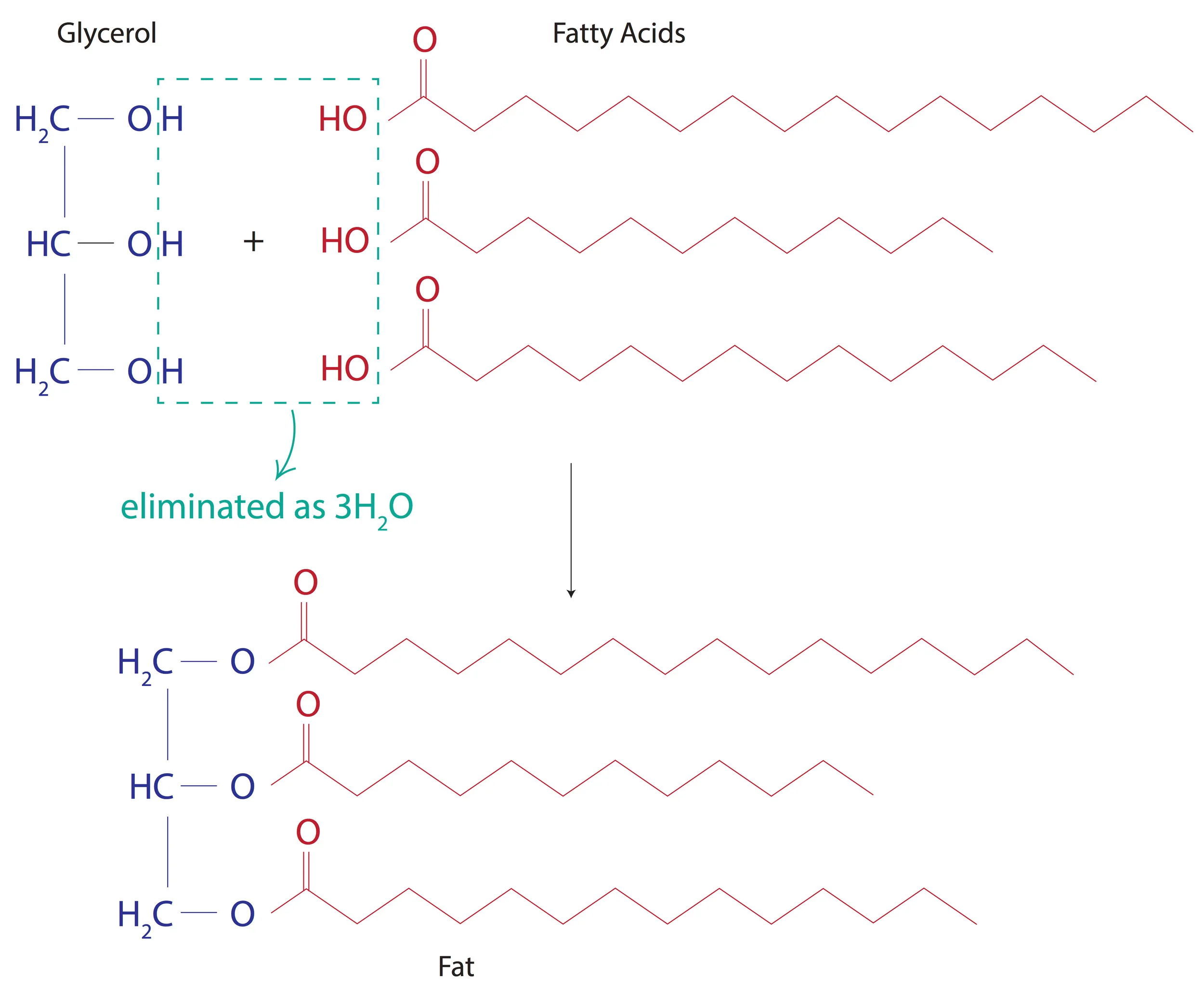
Fat is the simplest of all lipids. It’s an ester of fatty acid with glycerol. Yeah, it might seem hard. So let’s break it down.
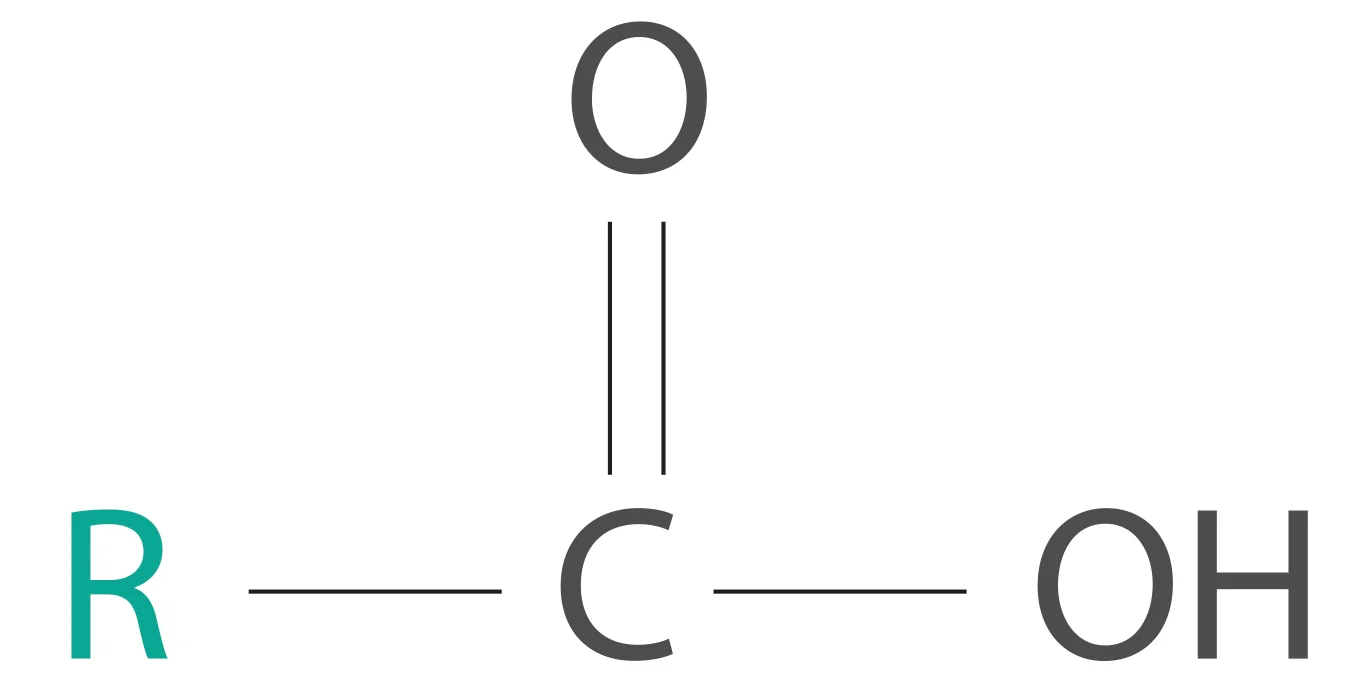
We all know what a carboxylic acid is.
A carboxylic acid
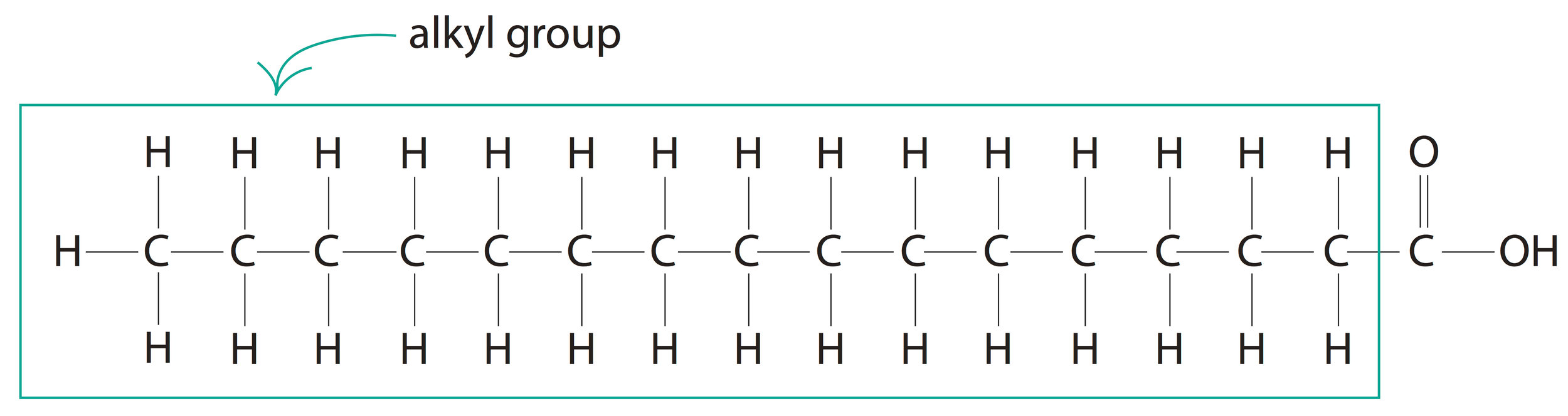
This R (alkyl) group in a carboxylic acid could contain any number of carbon atoms. When the total number of carbon atoms in a carboxylic acid (including the carboxyl group) exceeds four, we will call it a fatty acid. A good example would be the 16-carbon palmitic acid [CH3(CH2)14COOH].
Palmitic Acid
The significance of the number of carbon atoms in any fatty acid is that, the melting point of a fatty acid increases with increase in the number of carbon atoms, i.e. greater the number of carbon atoms, higher is the melting point of the fatty acid and hence, more solid is the fatty acid at room temperature. Since fatty acids are the building blocks of lipids such as fats, cholesterol and lipoproteins, they also take up the physical characteristics of fatty acids.
A little more should we know about fatty acids. As seen in the above structure of palmitic acid, all bonds in the alkyl group (hydrocarbon chain) are single. So, palmitic acid is an example of a saturated fatty acid. When one or more double bonds are introduced between carbon atoms in the hydrocarbon chain we will call it a mono or polyunsaturated fatty acid respectively. An example of a mono unsaturated fatty acid is Palmitoleic acid and an example of a poly unsaturated fatty acid is Linoleic acid.
Palmitoleic acid (16 C)(1 double bond)
Linoleic acid (18 C)(2 double bonds)
To make this clumsy structure pleasant to our eyes, hereafter I will represent fatty acids as a zigzag line in which the corners represent a carbon atom whose valencies are fulfilled by hydrogen atoms unless otherwise specified. Redrawing all the structures we have seen, we now have:
Palmitic acid
Palmitoleic acid
Linoleic acid
So what does the introduction of a double bond actually do to a fatty acid?
And why are Poly Unsaturated Fatty Acids considered healthier than Saturated Fatty Acids?
There are two reasons:
1) For every double bond we introduce into a fatty acid, we reduce its melting point to a certain extent. Remember, even complex lipids (made of fatty acids) take up this property of fatty acids. The lipids in our body or any organism for that matter, have to exist as a fluid. This is one of the reasons for the health benefits of poly-unsaturated fatty acids (PUFA) which exist as liquids at physiological temperature. Organisms like fish which live in the cold environment of the sea also have to maintain their lipids in the fluid state. How do they do that? They make their fatty acids as much poly-unsaturated as they can. Thus fish oils are rich in PUFA.
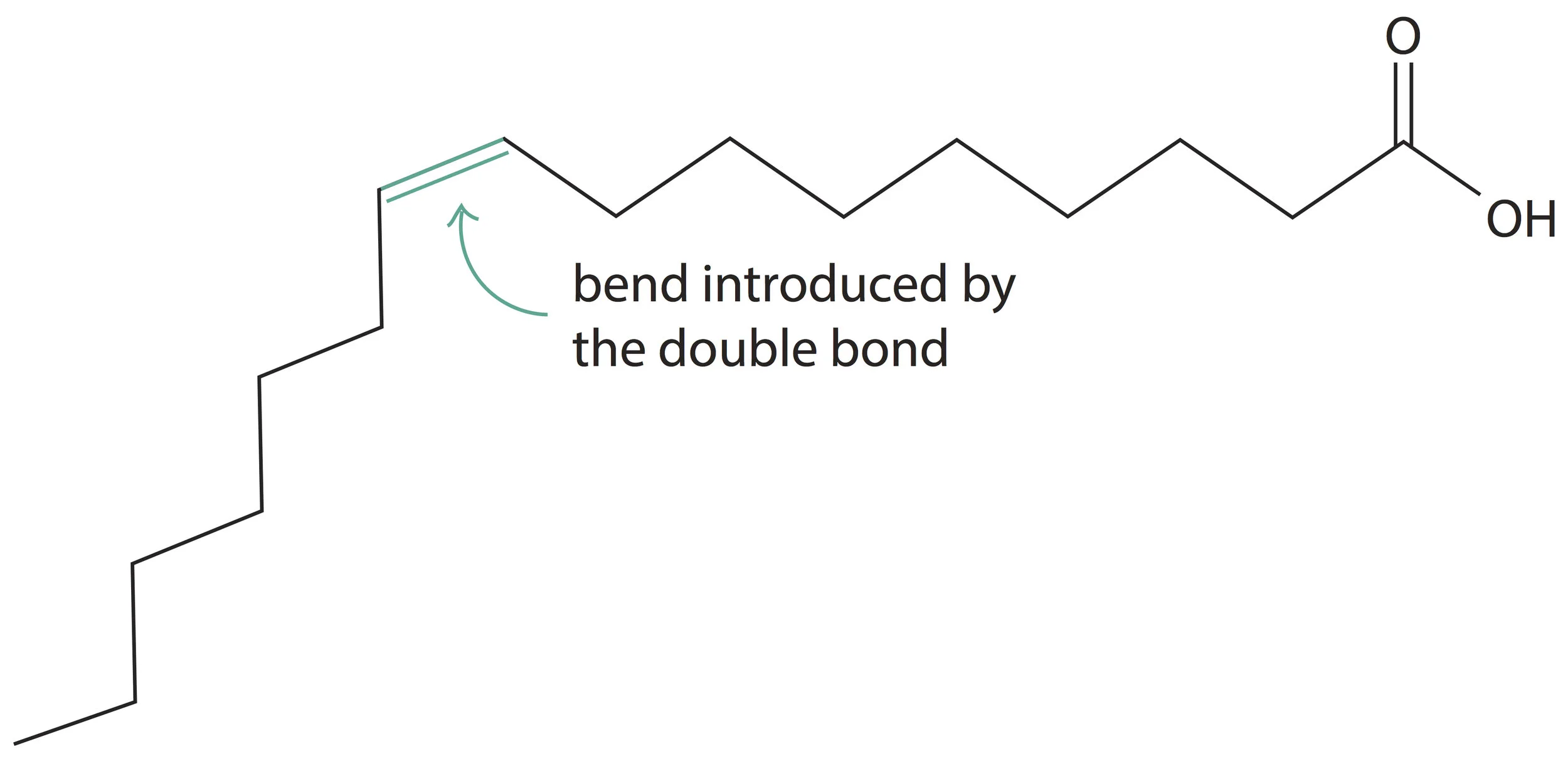
2) Another effect of adding a double bond to a fatty acid is to introduce a bend (kink) in the straight hydrocarbon chain. For example, the real structure of palmitoleic acid which we discussed before will be:
Palmitoleic acid
Now, imagine a fatty acid with numerous double bonds like arachidonic acid:
This property of unsaturated fatty acids help them to attain a compact structure occupying lesser space in the thin biological membranes. If this property had not been there, all fatty acid chains would be protruding out from the cell membranes leading to their instability.
So, more unsaturated a fatty acid is, better it is for us.
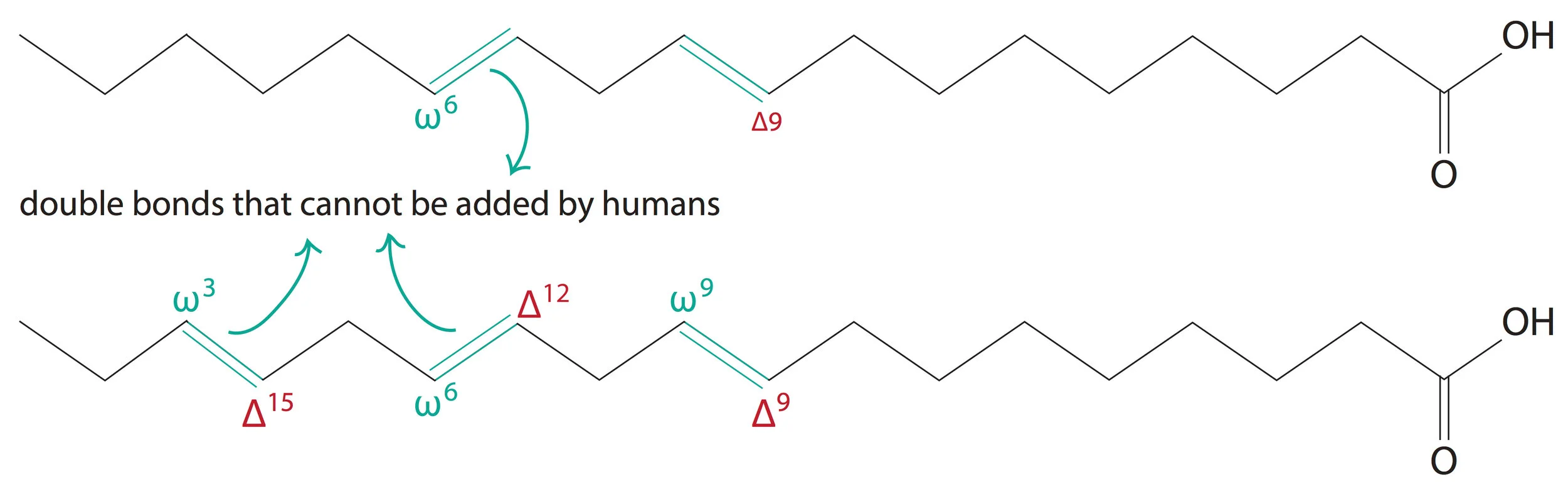
Another important thing that we are supposed to know regarding fatty acids is the numbering of the carbon atoms in a chain. This is so simple. There are two systems of numbering. One from each end of the fatty acid chain.
The delta(Δ) system starts numbering from the carbon atom of the carboxyl group. The omega(ω) system starts numbering from the carbon atom on the other end of the hydrocarbon chain.
The double bond in the shown fatty acid chain would be denoted as a double bond at the Δ-9 or the ω-7 carbon atom.
We humans lack the desaturase enzymes used to add double bonds beyond the Δ-9 carbon atom in any fatty acid. So, a fatty acid that has one or more double bonds beyond the Δ9 carbon atom (Δ-12 or Δ-15 for example) cannot be synthesized by us and hence has to be taken in via diet (essential fatty acid). Two such examples are linoleic and α-linolenic acids.
This much on fatty acids is enough for a better understanding on lipids. Remember, we started all this when we were trying to make fat from fatty acids.
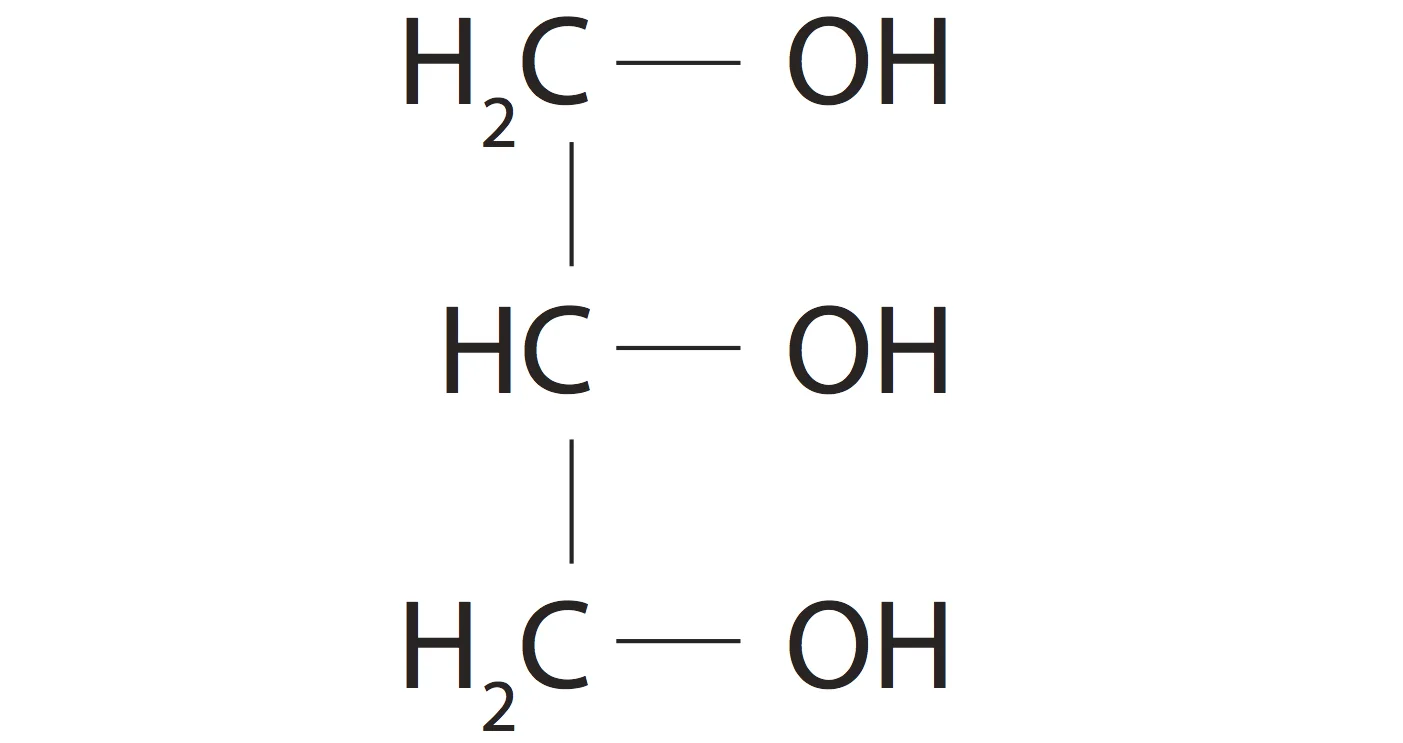
Glycerol is an alcohol whose structure we should know.
Fats are formed when the alcohol group(-OH) of glycerol is esterified with the carboxy group(-COOH) of fatty acids.
Yay! We’ve made a fat finally. A fat in its liquid form is called an oil.
I suppose this much is essential for the further understanding of complex lipids and their metabolism which will be covered in future articles.
Author: Anten (Facebook)
Sources and citations
Botham, Kathleen M., and Peter A. Mayes. "Lipids of Physiologic Significance." Harper's Illustrated Biochemistry. 30th ed. New York: McGraw-Hill Education, 2015. 212-15. Print.