Iron Metabolism - The story of a shopaholic
Iron is one of the most necessary metals in our body. It forms a vital part of some incredibly complicated and essential structures which permit life. However, despite being so important to our everyday survival, iron is not something that can be synthesised in our body, which means it needs to come from outside our body.
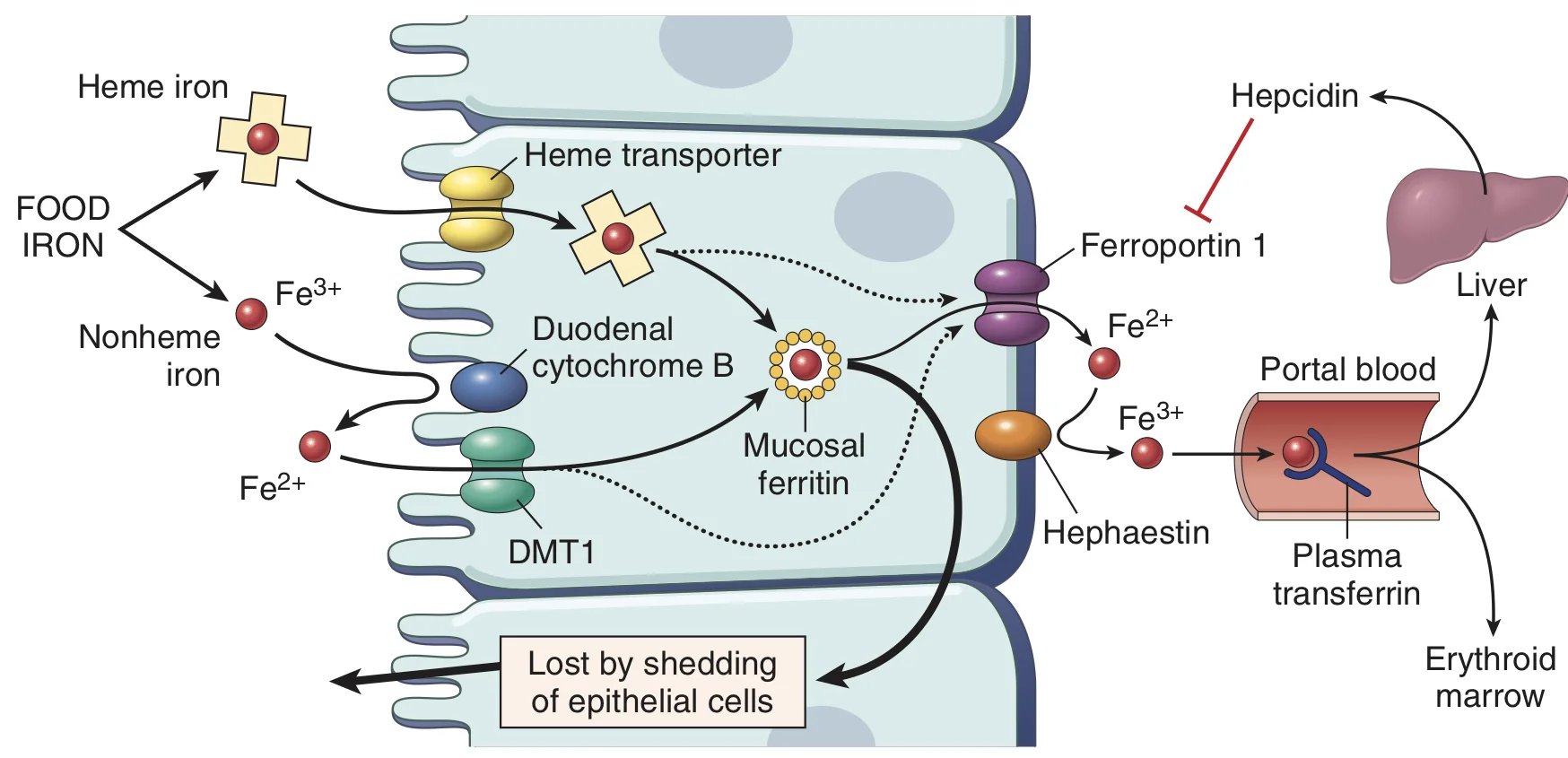
Now as far as medicine goes, iron is present in two dietary forms, heme iron and non-heme iron. Heme iron is the iron which is contained within a protoporphyrin ring as in hemoglobin or myoglobin. Non-heme iron is iron that is present in a more free form of salts. Because it’s already housed in an organic compound, heme iron is more readily absorbed than non heme iron.
So let’s understand how iron weaves it’s way into our bodies.
Elementary iron is available in two forms: ferrous (Fe 2+) and ferric (Fe 3+). The ferrous form is more friendly. It’s also the only form that is absorbed from the intestine because the intestines have a single ion transporter for many ionic compounds and that transporter only transports divalent ions. In fact, the transporter is called DMT -1 (divalent metal ion transporter -1).
So what happens to the ferric ions? As our body needs iron, it does not waste any of the iron that is made available to it. In the intestinal lumen, ferric ions are converted into ferrous ions by an enzyme called ferroreductase. This way all the iron is converted to ferrous form and is made available for absorption.
Now, the brush border cells of the small intestine have DMT -1which works to absorb the iron into the intestinal cells. From here, the iron can either get stored or it can get pushed into the blood where it can be distributed to the rest of the body.
Before we talk about the rest of iron metabolism, let’s understand something. Iron is like a shopaholic with a credit card. If iron is left alone, it can get really expensive for the body because free iron is toxic. So, just like shopaholics need people to accompany them to prevent them from buying expensive things like gold plated shoelaces, iron too, needs to be chaperoned. So everywhere in the body, iron is always accompanied by a protein - in case of transport and in case of storage. So let’s see the rest of the shopaholic’s story.
From the intestinal cell, the iron is pushed out into the blood by a transporter called ferroportin. As soon as the iron leaves the cell, it’s bound to a protein called Transferrin. Now transferrin is a bilobed glycoprotein that normally binds two molecules of iron. Once the iron is bound to it, the iron-transferrin complex binds to transferrin receptors on cell surfaces. The complex is then internalised by clathrin coated pits.
Inside the cell, the iron-transferrin-receptor complex is housed in an acidic vacuole. The low pH allows the iron to dissociate from the rest of the complex. Following the dissociation, the iron is used for heme synthesis and other functions, the transferrin- receptor complex is recycled back to the surface.
Once the iron becomes excessive, it is stored in the cell with another glycoprotein called ferritin.
Courtesy: Robbins Basic Pathology (9th Edition)
Now you know how iron is absorbed. Well, if something gets absorbed, then it must get excreted right? Well, not always. Iron does not have an exclusive mechanism of excretion. In case of iron excess, iron simply just doesn’t get absorbed anymore. This regulation of iron absorption is under the control of a hormone called Hepcidin. Remember that in order for iron to enter the blood from the intestinal cell the iron needed a protein called ferroportin? Well, hepcidin has a simple job: it inhibits ferroportin. This causes iron to be trapped inside the intestinal cell and when the cell is shed off, the iron is lost along with it.
The only other way iron is lost from the body is through blood loss. During blood loss, hemoglobin is lost and in the process iron bound to it is lost as well.
So that’s the metabolism of iron in a nutshell. Now as with every other metabolism, we study this one to better understand the problems that happen when the metabolism is impaired.
Let’s look at two conditions-
Anemia of chronic inflammatory disease and
Hemochromatosis - in which the metabolism of iron itself is impaired
In chronic inflammation, the hepcidin levels are high. This inhibits ferroportin which means that iron cannot be absorbed or mobilised from the stores. This results in a microcytic hypochromic anemia in which serum iron is low but ferritin levels are normal or high which signifies that serum iron is low despite good stores.
In the opposite end, we have hemochromatosis. There are many forms of hemochromatosis, both genetic and acquired. In one of the forms of hemochromatosis, hepcidin is low. This causes the ferroportin channel to be unchecked and allows excess iron to be absorbed. The issue with this is that, transferrin, the protein that binds iron in the blood, gets saturated eventually. And so free iron is found in the blood ready to wreak havoc. The excess iron gets deposited in various sites causing a range of symptoms from visual disturbances to cardiac anomalies.
The idea behind understanding the story of this shopaholic is not just to understand the disorders of its metabolism. The goal is to set a base for further understanding of an umbrella group of diseases called anemias which are characterised by low hemoglobin levels. So stay tuned for more!
Author: Narendran Sairam (Facebook)
Sources and citations
Brown, Robert H, et al. “Iron deficiency Anemia.” Harrison's Principles of Internal Medicine, by Larry Jameson et al., 19th ed., vol. 2, McGraw Hill, 2017